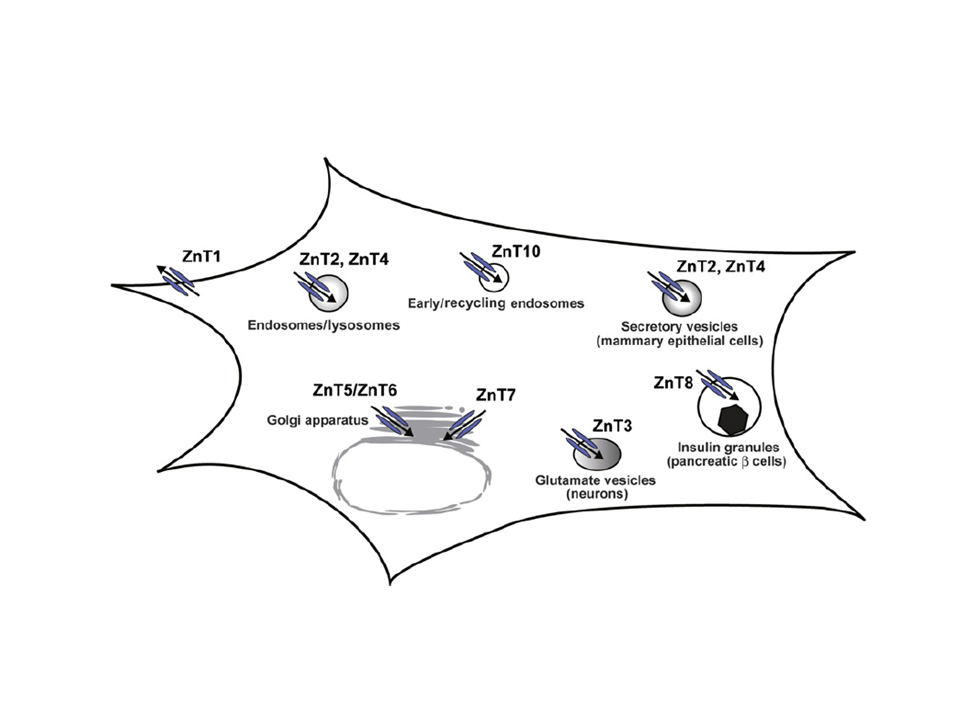
Zinc transport by Cation Diffusion Facilitators
Ten percent of proteins are estimated to bind zinc. In particular, zinc plays essential roles as cofactor for catalytic and redox enzymes as well as a structural element for transcription factors. Zinc is found at high levels in certain organelles, such as secretory vesicles associated with insulin, glutamanergic synapses, mother’s milk, and semen. The immune response is strongly dependent on zinc and so-called zincosomes produce a dramatic zinc “spark” that prevents polyspermy during fertilization of vertebrate eggs. Although the total concentration of Zn is > 0.1 mM, essentially all of it is bound either by protein or cytoplasmic chelating agents. The distribution of Zn within the cell is controlled by a network of transporters from several different protein families. In humans, export of Zn from the cytoplasm is the responsibility of proteins from the Cation Diffusion Facilitator family, named Znt1-10. They are accompanied by the ZIP family, generally responsible for import of Zn into the cell, and by ABC transporters and P-type pumps in some situations.
Bacterial Zn transporter YiiP
YiiP a bacterial member of the Cation Diffusion Facilitator family. We study the protein from Shewanella oneidensis, which is highly homologous to the protein from E. coli. Like most members of the family, YiiP forms a homodimer with six transmembrane helices and a C-terminal domain in the cytoplasm. YiiP operates as a Zn/H antiporter, meaning that it exchanges Zn(2+) ions with protons (H+) and therefore can use the proton-motive force as an energy source to drive transport. YiiP has conserved Zn-binding sites (pink spheres) within the transmembrane domain (site A) and the C-terminal domain (site C). In addition, YiiP has a non-conserved site in a loop between transmembrane helices 2 and 3 (site B). One of our main interests is to understand the role of these individual sites in transport.
-

Tubular membrane crystals
Initial structures were determined after reconstituting YiiP into a lipid bilayer and inducing formation of helical arrays
-

Complex of YiiP and Fab
Recent work has employed single particle analysis of a complex between YiiP and an Fab antibody fragment, producing isolated Y-shaped particles
-

The conformation of the YiiP dimer is the same in lipid bilayers and in detergent
-

Blue elements belong to YiiP, whereas the orange and red elements belong to the Fab
Crosslinking to probe conformational change
Cryo-EM structures both in lipid membranes and in detergent micelles have compact transmembrane domains (blue structure), whereas the initial X-ray structure from the Fu laboratory (pink structure) showed transmembrane domains in a splayed conformation. This comparison suggested that transport might be accompanied by a scissoring movement. To test this idea, we engineered cysteine residues at four sites along the dimer interface (yellow spheres) and used copper to induce crosslinking of the dimer (band marked “D” on the gel comparing a Cys-free construct, C190A, and four different mutants) . Assays of transport indicate that the crosslinking had no effect, suggesting that this conformational change is not essential for transport. The D51A and K79D mutations inactivate YiiP and served as negative controls for transport.
Characterization of individual Zn binding sites
-

Cryo-EM structure indicates that mutation of site A to prevent Zn binding fails to produce any conformational change. Microscale thermophhoresis (MST) shows that site A has a large dependence on pH (3.5 nM at pH 7 and 200 uM at pH 5.6)
-

Cryo-EM structure illustrates an asymmetrical conformational change induced by mutation at site B leading to an occluded state. MST shows that site B has an affinity of ~5 uM with minimal dependence on pH.
-

Cryo-EM structure reveals a dramatic reconfiguration of the YiiP dimer into a domain-swapped tetramer due to mutations at site C. This bi-nuclear site has moderate pH dependence with affinities ranging from 35 nM at pH 7 to 4 uM at pH 5.6.
Read More
Hussein A, Fan S, Lopez-Redondo M, Kenney I, Zhang X, Beckstein O, Stokes DL. Energy Coupling and Stoichiometry of Zn2+/H+ Antiport by the Cation Diffusion Facilitator YiiP. BioRxiv. 2023. doi: https://doi.org/10.1101/2023.02.23.529644.
Lopez-Redondo M, Fan S, Koide A, Koide S, Beckstein O, Stokes DL. Zinc binding alters the conformational dynamics and drives the transport cycle of the cation diffusion facilitator YiiP. J Gen Physiol. 2021 Aug 2;153(8). doi: 10.1085/jgp.202112873.
Lopez-Redondo ML, Coudray N, Zhang Z, Alexopoulos J, Stokes DL. Structural basis for the alternating access mechanism of the cation diffusion facilitator YiiP. Proc Natl Acad Sci U S A. 2018 Mar 20;115(12):3042-3047. doi: 10.1073/pnas.1715051115.
Coudray N, Valvo S, Hu M, Lasala R, Kim C, Vink M, Zhou M, Provasi D, Filizola M, Tao J, Fang J, Penczek PA, Ubarretxena-Belandia I, Stokes DL. Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy. Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2140-5. doi: 10.1073/pnas.1215455110.